p-Iodobenzaldehyde is elaborated to the fluorous alcohol p-Rf8(CH2)3C6H4CH(OH)(CH2)2Rf8 (three steps/80%; Rf8 = n-C8F17), which is converted to imine p-Rf8(CH2)3C6H4C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(CH2)3Rf8)(CH2)2Rf8 (6, two steps/93%) and thioether p-Rf8(CH2)3C6H4CH(S(CH2)3Rf8)(CH2)2Rf8 (12, 64%). Reactions with Pd(OAc)2 (AcOH, 95 °C) give palladacycles with [RC
N(CH2)3Rf8)(CH2)2Rf8 (6, two steps/93%) and thioether p-Rf8(CH2)3C6H4CH(S(CH2)3Rf8)(CH2)2Rf8 (12, 64%). Reactions with Pd(OAc)2 (AcOH, 95 °C) give palladacycles with [RC![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) 6H3CR′
6H3CR′![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(R)Pd
N(R)Pd![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) (μ-OAc)]2 (7, 87%) and [RC
(μ-OAc)]2 (7, 87%) and [RC![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) 6H3CHR′S(R)Pd
6H3CHR′S(R)Pd![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) (μ-OAc)]2 (13, 84%) cores. The former reacts with LiCl and LiI to give the corresponding bridging halide complexes (8, 9); LiCl/PPh3 affords monomeric RC
(μ-OAc)]2 (13, 84%) cores. The former reacts with LiCl and LiI to give the corresponding bridging halide complexes (8, 9); LiCl/PPh3 affords monomeric RC![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) 6H3CR′
6H3CR′![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(R)Pd
N(R)Pd![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) (Cl)(PPh3) (10). Palladacycles 7–9 and 13 are poorly soluble or insoluble in many solvents at 20–24 °C, but much more soluble at higher temperatures. The CF3C6F11/toluene partition coefficients of 6, 7, 12, and 13 are >91∶<9 (24 °C). Both 7 and 13 are excellent catalyst precursors for Heck reactions of aryl halides. Turnover numbers exceed 106 with phenyl iodide under homogeneous conditions in DMF at 140 °C. The palladacycles precipitate as bridging halides upon cooling, and can in theory be recovered by liquid/solid phase separations. However, since the quantities are small, the solvent C8F17Br is added for recycling. Induction periods in both the first and second cycles, and progressively lower activities, are noted. Transmission electron microscopy indicates the formation of soluble palladium nanoparticles. Together with other data, it is proposed that the nanoparticles are the active catalysts, for which the recyclable palladacycles constitute a steady state source, until exhausted. Complex 7 similarly catalyzes the Suzuki reaction (K3PO4, toluene, 130 °C).
(Cl)(PPh3) (10). Palladacycles 7–9 and 13 are poorly soluble or insoluble in many solvents at 20–24 °C, but much more soluble at higher temperatures. The CF3C6F11/toluene partition coefficients of 6, 7, 12, and 13 are >91∶<9 (24 °C). Both 7 and 13 are excellent catalyst precursors for Heck reactions of aryl halides. Turnover numbers exceed 106 with phenyl iodide under homogeneous conditions in DMF at 140 °C. The palladacycles precipitate as bridging halides upon cooling, and can in theory be recovered by liquid/solid phase separations. However, since the quantities are small, the solvent C8F17Br is added for recycling. Induction periods in both the first and second cycles, and progressively lower activities, are noted. Transmission electron microscopy indicates the formation of soluble palladium nanoparticles. Together with other data, it is proposed that the nanoparticles are the active catalysts, for which the recyclable palladacycles constitute a steady state source, until exhausted. Complex 7 similarly catalyzes the Suzuki reaction (K3PO4, toluene, 130 °C).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(CH2)3Rf8)(CH2)2Rf8 (6, two steps/93%) and thioether p-Rf8(CH2)3C6H4CH(S(CH2)3Rf8)(CH2)2Rf8 (12, 64%). Reactions with Pd(OAc)2 (AcOH, 95 °C) give palladacycles with [RC
N(CH2)3Rf8)(CH2)2Rf8 (6, two steps/93%) and thioether p-Rf8(CH2)3C6H4CH(S(CH2)3Rf8)(CH2)2Rf8 (12, 64%). Reactions with Pd(OAc)2 (AcOH, 95 °C) give palladacycles with [RC![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) 6H3CR′
6H3CR′![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(R)Pd
N(R)Pd![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) (μ-OAc)]2 (7, 87%) and [RC
(μ-OAc)]2 (7, 87%) and [RC![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) 6H3CHR′S(R)Pd
6H3CHR′S(R)Pd![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) (μ-OAc)]2 (13, 84%) cores. The former reacts with LiCl and LiI to give the corresponding bridging
(μ-OAc)]2 (13, 84%) cores. The former reacts with LiCl and LiI to give the corresponding bridging ![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) 6H3CR′
6H3CR′![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(R)Pd
N(R)Pd![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) (Cl)(PPh3) (10). Palladacycles 7–9 and 13 are poorly soluble or insoluble in many
(Cl)(PPh3) (10). Palladacycles 7–9 and 13 are poorly soluble or insoluble in many 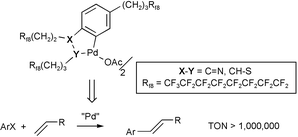

 Please wait while we load your content...
Please wait while we load your content...