Arsenic trioxide triggered calcium homeostasis imbalance and induced endoplasmic reticulum stress-mediated apoptosis in adult rat ventricular myocytes†
Abstract
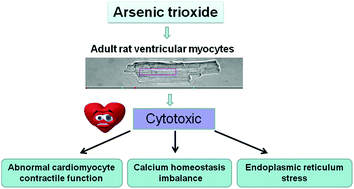
Arsenic trioxide (ATO) is a potent anticancer drug agent but its clinical use is often limited by severe cardiotoxicity. However, its exact mechanism remains poorly understood. In this study, we simultaneously explored the direct effect of ATO on cardiac contraction in adult rat ventricular myocytes and its effects on Ca2+ transient in real time by using an IonOptix MyoCam system. The results showed that ATO increased the amplitude of sarcomere shortening, the maximal velocity of relengthening and shortening (−dL/dtmax and +dL/dtmax), time-to-90% relengthening (TR90), and time-to-peak shortening (TPS), resulting in abnormal cardiomyocyte contraction. Meanwhile, ATO markedly increased the resting Ca2+ ratio, amplitude/resting calcium, the maximal velocity of Ca2+ shortening and relaxation (+d[Ca2+]/dtmax and −d[Ca2+]/dtmax), time-to-50% peak [Ca2+]i and the decay rate of [Ca2+]i transients, suggesting that ATO leads to intracellular imbalance of calcium homeostasis. ATO also inhibited sarcoplasmic reticulum Ca2+-ATPase 2a (SERCA2a) activity in a time-dependent manner and activated the endoplasmic reticulum (ER) stress reaction. These results revealed that ATO dramatically aggravates Ca2+ overload and promotes ER stress, eventually causing abnormal cardiomyocyte contraction in a dose-dependent and time-dependent manner.

 Please wait while we load your content...
Please wait while we load your content...