Reverse synthesis of CsPbxMn1−x(Cl/Br)3 perovskite quantum dots from CsMnCl3 precursors through cation exchange†
Abstract
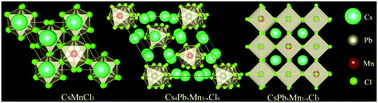
Successful development of all-inorganic CsPbX3 (X = Cl, Br, or I) perovskite quantum dots (PQDs) has been witnessed due to their unique optical and electrical properties. However, the heavy usage of Pb element in PQDs has limited the commercial application of these PQDs, and the preparation of low-Pb or Pb-free PQDs is imperative. In this study, we report room-temperature reverse synthesis of CsPbxMn1−xCl3 PDQs from CsMnCl3 precursor nanocrystals through cation exchange. Interestingly, two-step phase transformation processes from hexagonal CsMnCl3 to rhombohedral Cs4PbxMn1−xCl6 and finally to cubic CsPbxMn1−xCl3 are evidenced. With an increase in the reaction time, Mn2+ ions in the PQD lattice are gradually replaced by Pb2+ ions. Notably, Mn2+ d → d broadband luminescence is not detected for the Cs4PbxMn1−xCl6 product, but it is intense for the CsPbxMn1−xCl3 sample owing to efficient energy transfer from CsPbCl3 to Mn2+. Moreover, it is found that the introduction of Br− into the reaction system will prohibit the complete phase transformation from rhombohedral Cs4PbxMn1−x(Cl/Br)6 to cubic CsPbxMn1−x(Cl/Br)3. By controlling the ratio between the CsMnCl3 precursor and PbBr2, tunable luminescence is easily achieved due to the combined emissions from both CsPb(Cl/Br)3 PQDs and Mn2+-emitting centers.


 Please wait while we load your content...
Please wait while we load your content...