Selenium-containing BODIPY dyes as photosensitizers for triplet–triplet annihilation upconversion†
Abstract
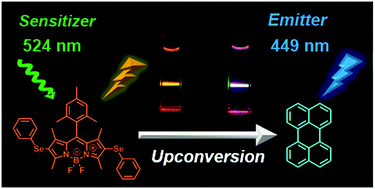
In this study, two new selenium-containing BODIPY dyes were synthesized as photosensitizers for triplet–triplet annihilation upconversion (TTA-UC). Bis(phenylselanyl)-BODIPY 1 absorbed visible light at 518 nm (εmax = 7.84 × 104 cm−1 M−1) and exhibited fluorescence at 594 nm with a low fluorescence quantum yield (ΦF) of 0.03. The low ΦF value was ascribed to heavy atom effects. When a TTA-UC system comprising 1 and the triplet acceptor perylene was set up, a purplish white emission was observed with a significant anti-Stokes shift of 77 nm and a high UC quantum yield (ΦUC′) of 17% in CH2Cl2. Notably, the intramolecular charge transfer of 1 enabled us to tune the emission color of the TTA-UC system. On the other hand, the use of selenophene derivative 2 as a sensitizer instead of 1 led to a decrease in the UC efficiency. The relationship between structure and sensitizer properties in the TTA-UC system is discussed herein.


 Please wait while we load your content...
Please wait while we load your content...