α,β-Unsubstituted meso-positioning thienyl BODIPY: a promising electron deficient building block for the development of near infrared (NIR) p-type donor–acceptor (D–A) conjugated polymers†
Abstract
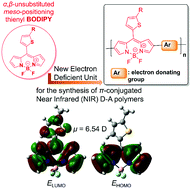
It is demonstrated that α,β-unsubstituted meso-positioning thienyl BODIPY is an electron deficient unit that leads to the development of ultra low optical band gap (Eoptg < 1 eV) π-conjugated D–A quarterthiophene polymers. Furthermore, it is revealed that the optoelectronic, electrochemical and charge transporting properties of the resulting α,β-unsubstituted meso-positioning thienyl BODIPY quaterthiophene-based polymers are alkyl side chain positioning dependent. Tail-to-tail (TT) positioning of the alkyl side chains at the two central thiophenes of the quaterthiophene segment results in lower Eoptg, higher energy levels and increased hole mobility as compared to head-to-head (HH) positioning. Finally, even though the synthesized polymers exhibit high electron affinity, higher even than that of the fullerene derivative [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM), they present only p-type behaviour in field effect transistors (FETs) independent of the alkyl side chain positioning.


 Please wait while we load your content...
Please wait while we load your content...