Fluorination increases the electron mobility of zinc azadipyrromethene-based electron acceptors and enhances the performance of fullerene-free organic solar cells†
Abstract
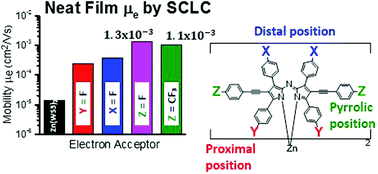
Structure–property studies were performed on a series of four fluorinated zinc azadipyrromethene derivatives. This series is based on bis[2,8-diphenylethynyl-1,3,7,9-tetraphenylazadipyrromethene]zinc(II) (Zn(WS3)2), with fluorine atoms at the para-position of either the proximal phenyls, the distal phenyls or the pyrrolic phenylethynyls, refered to as the proximal, distal and pyrrolic positions, respectively. In order to study the degree of fluorination, we also added –CF3 to the pyrrolic positions. All complexes had similar absorption spectra, 600–800 nm in films, complementing the absorption of the well-known donor poly(3-hexylthiophene) (P3HT). The chelates were tested in bulk heterojunction organic photovoltaic (OPV) devices using P3HT as the electron donor. Compared to the unfluorinated acceptor Zn(WS3)2, fluorination increased the power conversion efficiencies (PCEs) in all cases except in the proximal position. The best results were obtained when either F or CF3 was added to the pyrrolic positions with PCEs of 3.3% and 3.7%. Atomic force microscopy images revealed a favorable phase separation and showed no signs of large-scale aggregation for all blends. Light intensity measurements revealed that bimolecular recombination limits the performance in these fullerene-free devices, and that the addition of fluorine suppressed the bimolecular recombination, with the largest suppression seen with the pyrrolic substitutions F and CF3. Electron mobility increased with fluorination, again with the largest increase when fluorines were added to the pyrrolic positions, reaching mobilities as high as ∼10−3 cm2 V−1 s−1, on a par with electron mobility of the ubiquitous phenyl-C61-butyric acid methyl ester (PCBM) acceptor in blends. These results point to the importance of the chemical composition of pyrrolic phenylethylnyls for optimizing the charge transport and device performance for these types of complexes.


 Please wait while we load your content...
Please wait while we load your content...
