The design of smectic liquid crystals with an axially chiral biphenyl core: in search of a proper ferroelectric liquid crystal phase†
Abstract
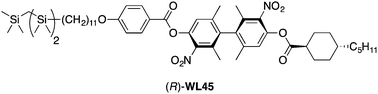
We report the results of a study in which we introduce a tricarbosilane end-group in a mesogenic scaffold derived from an axially chiral biphenyl with a large transverse dipole moment. This structural modification is intended to promote the formation of a chiral smectic C (SmC*) phase, with the aim of discovering a mesogen with a virtual transition temperature to a proper ferroelectric phase (To) that approaches the crystal-SmC* melting point. We show herein that the combination of a 4-pentylbicyclo[2.2.2]octane-1-carboxylate chain and a tricarbosilane-terminated 4-alkoxybenzoate chain gives a mesogen (R,S)-WL43 forming an enantiotropic orthogonal smectic A (SmA) phase and a higher order tilted smectic phase. Substitution of the 4-pentylbicyclo[2.2.2]octane-1-carboxylate group with a trans-4-pentylcyclohexane-1-carboxylate group gives a mesogen (R,S)-WL45 forming monotropic SmA and SmC phases. The SmC* phase formed by the enantiomerically pure (R)-WL45 exhibits Goldstone-mode switching characteristic of a surface-stabilized FLC, but its spontaneous polarization PS is too low to be measured by conventional methods.


 Please wait while we load your content...
Please wait while we load your content...