Kinetically controlled synthesis of Cu nanowires with tunable diameters and their applications in transparent electrodes†
Abstract
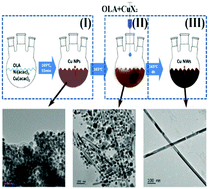
One-dimensional metallic nanostructures have attracted ever increasing attention in recent years due to their potential applications in transparent electrodes, catalysts, surface enhanced resonance scattering (SERS) based sensors and electric heating elements. The controllable synthesis of high quality metal nanowires is of great importance for the optimization of devices based on metal nanowires. However, due to the intrinsic complexity of one-pot organic reaction systems, the reaction mechanism is still yet to be understood, which severely holds back the efforts towards the controllable synthesis of high quality nanowires. Herein, a two-step method for the synthesis of high-quality Cu nanowires with tunable diameters is proposed. The mechanism of a typical synthesis procedure is discussed through analyzing the apparent phenomena and intermediate products. Kinetically controlled synthesis is achieved by adjusting the species and concentration of halide ions to synthesize high-purity Cu nanowires with tunable diameters ranging from 20 to 90 nm. Electrodes with superior conductivity (FoM ∼ 140, 11 Ω sq−1@80%) are constructed and characterized on the basis of the Cu nanowires synthesized through this method. Red shifts in the LSPR peaks of Cu nanowire dispersions and increments in the haze factors of nanowire electrodes are observed as the average diameter of Cu nanowires increases. The method is also successfully expanded to the synthesis of Ag nanowires with tunable diameters.


 Please wait while we load your content...
Please wait while we load your content...