In situ electrochromic efficiency of a nickel oxide thin film: origin of electrochemical process and electrochromic degradation†
Abstract
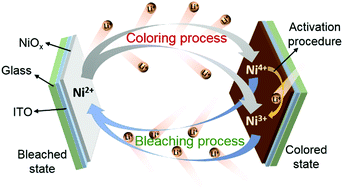
Electrochromic nickel oxide (NiOx) thin films are one of the most promising anodic colored materials. However, there is lack of accurate description of their electrochemical process and degradation mechanism. In this study, a novel approach involving in situ electrochromic efficiency is proposed to reveal the electrochemical origin of an electrochromic NiOx thin film cycled in a Li+-ion electrolyte. The results indicate that the coloring process of the NiOx thin film refers to the oxidation reactions of Ni2+ to Ni3+ and Ni2+ to Ni4+ (in two forms of Ni3O4 and Ni2O3), and the bleaching process is associated with the reduction reactions of Ni4+ to Ni2+, Ni4+ to Ni3+, and Ni3+ to Ni2+. The irreversible reduction of Ni4+ to Ni3+ plays a dominant role in the activation procedure of NiOx. It is deduced that the Li+-ion trapping in the bleaching process along with the reduction reactions of Ni4+ to Ni3+ and Ni3+ to Ni2+ causes the degradation of the electrochromic properties. This study provides a further insight into the electrochromic mechanism and is conducive to the improvement of the long-term cyclic durability for Li+-based electrochromic NiOx. Moreover, the study significantly establishes a direct connection between an electrochemical process and a variation in the optical absorbance of materials.


 Please wait while we load your content...
Please wait while we load your content...