An optoelectronic duple bistable phosphate with ultrahigh thermal stability†
Abstract
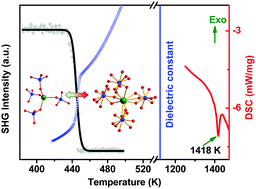
Bistable compounds have attracted intensive interest because of their great potential to realize seamless integration of multifunctional switches in one device. However, previous studies are mainly limited to organic–inorganic hybrid compounds, which have relatively low phase-transition temperature and thermal stability. Here, we report a new pure inorganic compound CsCdPO4, which undergoes a high-temperature phase transition at 447 K. Correspondingly, CsCdPO4 simultaneously shows an interesting duple bistability of nonlinear optical and dielectric properties. Furthermore, it shows an extremely high thermal stability up to 1418 K, which is significantly higher than those of organic–inorganic bistable compounds. This work will open up a new avenue to develop multifunctional materials that remain stable even under extreme operating conditions (e.g. extremes of temperature).


 Please wait while we load your content...
Please wait while we load your content...