Design, synthesis, photophysical properties and pH-sensing application of pyrimidine-phthalimide derivatives†
Abstract
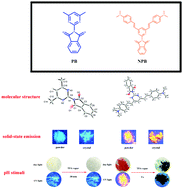
On the basis of the molecular design of the donor–π–acceptor (D–π–A) pyrimidine-phthalimide derivatives, two new atypical AIE chromophores, 2-(4,6-dimethylpyrimidin-2-yl)isoindoline-1,3-dione (PB) and 2-(4,6-bis(4-(dimethylamino)styryl)pyrimidin-2-yl)isoindoline-1,3-dione (NPB), were synthesized and characterized by using IR, 1H NMR, 13C NMR and HRMS. Both PB and NPB exhibited obvious solid-state fluorescence emission due to their twisted geometries and positive solvatochromism due to their different molecular conformations in various solvents. Owing to the different push–pull electronic effects of substituents on the pyrimidine moiety, PB and NPB, acting as D–π–A compounds, showed different HOMO–LUMO gaps and a variable red-shifted emission in their solid state, substantiating the possibility to tune effectively the photophysical properties of these AIE chromophores by rational molecular design. In addition, PB and NPB could be easily and reversibly protonated at the site of the nitrogen atoms, causing dramatic color changes. This phenomenon opens up the potential avenues of developing novel colorimetric pH sensors and logic gates for specific applications.


 Please wait while we load your content...
Please wait while we load your content...