Tuning of luminescence color of π-conjugated liquid crystals through co-assembly with ionic liquids†
Abstract
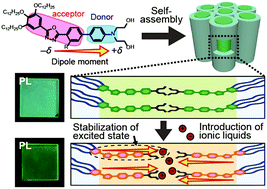
Luminescent oxadiazole-based columnar liquid crystals containing a diethanolamine moiety forming intramolecular donor–acceptor charge transfer states have been designed and synthesized. These molecules exhibit strong solvatochromism depending on the solvent polarity. The co-assembly of oxadiazole derivatives and nonvolatile 1-butyl-3-methylimidazolium-based ionic liquids through hydrogen bonding leads to the formation of thermally stable nanosegregated columnar structures with confined channels of ionic liquids. Modulation of the emission color from light-blue to blue-green in the liquid-crystalline states has been achieved by incorporating the ionic liquid. This red-shifted emission can be attributed to the stabilization of the excited intramolecular charge transfer state of the donor–acceptor luminophore by polar molecules. The presented tunable luminescent soft materials obtained through noncovalent supramolecular approaches will open a new avenue in the fields of optoelectronic organic materials.


 Please wait while we load your content...
Please wait while we load your content...