Sky-blue aggregation-induced emission molecules for non-doped organic light-emitting diodes†
Abstract
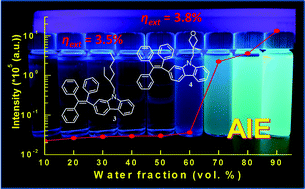
Triphenylethene moieties decorated with different carbazole-based substitutions were synthesized to realize molecules harvesting strong blue aggregation-induced emission (AIE). Among these compounds, 1,1,2-triphenyl-2-[9-(2-ethylhexyl)carbazol-2-yl]ethylene (3) and 1,1,2-triphenyl-2-[9-((3-methyloxetan-3-yl)methyl)carbazol-2-yl]ethylene (4) were found to possess suitable carrier transport capabilities, high thermal stability, and strong blue aggregation-induced emissions. In addition, bipolar charge transport for carbazole derivatives substituted with a triphenylethylene moiety was found. Both compounds 3 and 4 were selected as emitters to fabricate non-doped blue organic light-emitting diodes (OLEDs). The respective maximum efficiencies of compounds 3- and 4-based AIE OLEDs were recorded at 3.5% (8.8 cd A−1 and 8.5 lm W−1) and 3.8% (9.0 cd A−1 and 8.4 lm W−1), respectively. Furthermore, a red phosphorescent emitter was used to combine compounds 3 and 4 for white OLEDs, giving respective peak efficiencies of 2.8% and 2.7%. These results demonstrate the effective design concepts of the triphenylethene moiety decorated with different carbazole-based substitutions.


 Please wait while we load your content...
Please wait while we load your content...