Synthesis and magnetic characterisation of Fe1−xMgxSb2O4 (x = 0.25, 0.50, 0.75) and their oxygen-excess derivatives, Fe1−xMgxSb2O4+y
Abstract
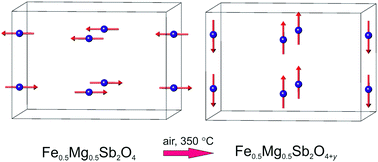
Three new materials of composition Fe1−xMgxSb2O4 (x = 0.25, 0.50, 0.75) with the tetragonal schafarzikite structure have been synthesised. Magnetic susceptibility measurements suggest that Fe1−xMgxSb2O4 (x = 0.25, 0.50) are canted antiferromagnets whilst Fe0.25Mg0.75Sb2O4 is paramagnetic. The magnetic ordering temperatures decrease as the Mg2+ concentration increases. The materials form oxygen-excess phases when heated in oxygen-rich atmospheres at temperatures of ∼350 °C. 57Fe Mössbauer spectroscopy shows that the oxidation process involves the oxidation of Fe2+ to Fe3+. Powder neutron diffraction confirms the location of the excess oxygen within the structural channels and reveals a change in magnetic order at low temperatures from A-type (magnetic moments along 〈100〉) for Fe1−xMgxSb2O4 to C-type (magnetic moments along [001]) for the oxidised materials. The change is attributed to a weakening of the antiferromagnetic exchange interactions between edge-linked FeO6 octahedra for the Fe3+-containing materials.


 Please wait while we load your content...
Please wait while we load your content...