Strong ferroelectric polarization of CH3NH3GeI3 with high-absorption and mobility transport anisotropy: theoretical study†
Abstract
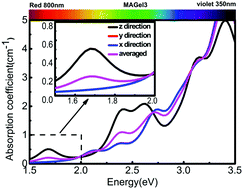
Lead iodide perovskite has long been considered to be promising for photovoltaic applications due to the high power conversion efficiency. However, the toxicity and instability of lead iodide perovskite limit its widespread use. Recently, germanium iodide perovskite CH3NH3GeI3 has attracted much attention due to the lead-free and high distorted structure. In this study, we exhibited a detailed theoretical investigation to predict total ferroelectric polarization of about 13.8 μC cm−2 and disentangle the contributions of MA cation (MA = CH3NH3), the center charge of MA cation and Ge2+ ion relative to the inorganic framework. Based on the electronic structure, optical absorption as well as carrier mobility of the system are further calculated, which exhibit strong anisotropy along the same direction. Further regulating atomic structure to strengthen or weaken the polarization, variable optical absorption spectra and hence tunable efficiencies are obtained. By analyzing the electronic structure with applied increased polarization, we conclude that polarization driven optical absorption can be attributed to an increased p–p orbital transition from I to Ge. In the case of CH3NH3GeI3, we discuss the relationship between ferroelectricity and photovoltaic performance, reveal the potential of utilizing polar material for higher solar conversion, and speculate that it is a general rule to raise the efficiency by enhancing the polarization.


 Please wait while we load your content...
Please wait while we load your content...