A–π–D–π–A carbazole derivatives with remarkable solvatochromism and mechanoresponsive luminescence turn-on
Abstract
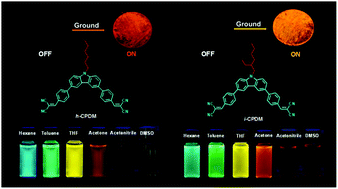
Two A–π–D–π–A molecules with a carbazole donor and a dicyanovinyl acceptor but differing in N-hexyl (h-CPDM) and N-isooctyl substituents (i-CPDM) were synthesized, and both of them presented remarkable dual properties of solvatochromism and mechanoresponsive luminescence (MRL) turn-on. The intrinsic intramolecular charge transfer (ICT) characteristic endowed both luminophors with a prominent solvatochromic effect, with emission color tuning from blue to orange-red by changing the solvent from nonpolar hexane to polar dimethyl sulfoxide. Meanwhile, the non-/weakly emissive original powders of h-CPDM and i-CPDM gave bright orange (610 nm) and yellow (596 nm) emission with the photoluminescence quantum yields increasing as high as 85-fold after being ground. Investigations revealed this mechanoresponsive luminescence turn-on could be ascribed to the disturbance of the π–π stacking interactions in the non-/weakly emissive J-aggregates by mechanical force. This work offers carbazole derivatives that can be used as sensitive fluorescent indicators for organic solvents and mechanical sensors.


 Please wait while we load your content...
Please wait while we load your content...