N-Annulated perylene diimide dimers: acetylene linkers as a strategy for controlling structural conformation and the impact on physical, electronic, optical and photovoltaic properties†
Abstract
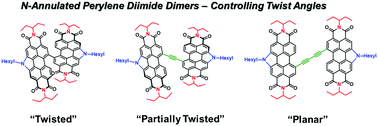
The geometry of organic π-conjugated small molecules can impact the morphology of blended-thin films and subsequent performance in opto-electronic devices. In this report, we investigate the role of molecular conformation of perylene diimide (PDI) dimers designed to act as non-fullerene acceptors in organic solar cells. A series of three PDI dimers is presented in which the PDI chromophores are directly linked via the bay position (PDI2, 3) or separated by one (PDI2Ac, 4) or two (PDI2Ac2, 5) acetylene spacers. In all cases, the exo-position of the PDI dimers is N-annulated. New compounds 4 and 5 were synthesized via an optimized and facile synthetic pathway. Directly linked PDI dimers adopted a highly twisted conformation whereas adding two acetylene spacers rendered the PDI chromophores coplanar. 1H NMR spectroscopic analysis of each dimer revealed a highly sensitive electronic structure that is strongly influenced by the acetylene spacers. It was found that compounds 4 and 5 with less twisted structures exhibited similar electron affinities but lower ionization potentials, lower organic solvent solubility, and red-shifted optical absorption spectra when compared to the highly twisted dimer 3. In addition, 4 and 5 showed a stronger tendency to aggregate in both solution and the solid state. This had a large impact on the performance of organic solar cells using these materials as electron acceptors. Bulk-heterojunction solar cells based upon a PTB7-Th:3 active layer could reach high power conversion efficiencies of 5.23%. In contrast, PTB7-Th:4 and PTB7-Th:5 based devices had ∼5 times lower performance owing to the formation of unfavourable active layer morphologies.
- This article is part of the themed collection: CSC100: Celebrating Canadian Chemistry


 Please wait while we load your content...
Please wait while we load your content...