Global minimum of two-dimensional FeB6 and an oxidization induced negative Poisson's ratio: a new stable allotrope†
Abstract
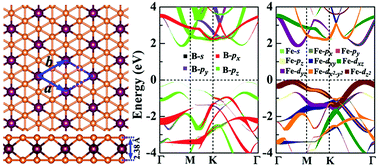
Using a global optimization evolutionary algorithm and density functional theory calculations we report, for the first time, two-dimensional tri-FeB6 as a global minimum ground state structure, opening the door for tailoring unique properties by manipulating transition metals. This novel tri-FeB6 consists of borophene-like layers and an Fe-layer with the Fe-layer being sandwiched between boron planes. Its dynamic stability, energetic stability, thermal stability, and mechanical stability have been carefully evaluated, suggesting it as a quite stable material to call for experimental realization. Furthermore, oxidization would retain the planar structural characteristics, resulting in an oxide sheet. Both tri-FeB6 and its oxide are found to have remarkable mechanical properties. In comparison with experimentally fabricated borophene, the in-plane stiffness in tri-FeB6 is greatly enhanced, showing the same stiffness as graphene. Oxidization brings about unusual negative Poisson's ratio properties, rendering the oxide sheet attractive in view of both scientific and technological investigations. In addition, both tri-FeB6 and its oxide are semiconductors with band gaps of about 2 eV. Furthermore, tensile strain can continuously tune the band gaps over a wide range, making tri-FeB6 and its oxide attractive for advanced nanoelectronics.


 Please wait while we load your content...
Please wait while we load your content...