Nine-ring angular fused biscarbazoloanthracene displaying a solid state based excimer emission suitable for OLED application†
Abstract
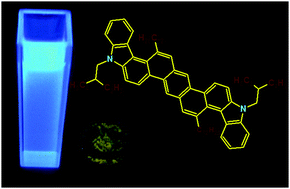
A new biscarbazoloanthracene consisting of nine fused aromatic rings, including two pyrrole units, has been obtained in a straightforward and convergent synthesis. Computational chemistry and conformational analysis revealed that the semiconductor's molecule is not planar, the two carbazole moieties being helical twisted from the plane of the anthracene unit. Photophysical and electrochemical measurements showed that this angular fused heteroacene has a low lying HOMO energy level with a wide band gap despite its extended π-conjugated molecular framework. Based on its relatively low-lying HOMO level, the semiconductor promises a high environmental stability in comparison to other related linear fused acenes and heteroacenes. The biscarbazoloanthracene has been applied as the light emitting layer in a white light emitting diode (WOLED). It is proposed that the white OLED feature is due to dual light emission properties from the active semiconductor layer being based on both the molecular luminescence of the small molecule and a discrete excimer emission made possible by suitable aggregates in the solid state. Noteworthy, this is the first reported example of such a behavior observed in a small molecule heteroacene rather than an oligomer or a polymer.


 Please wait while we load your content...
Please wait while we load your content...