Thermal stability and electrical conductivity of carbon-enriched silicon oxycarbide
Abstract
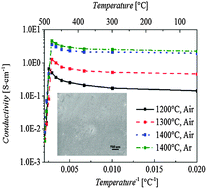
Silicon oxycarbide (SiOC) is an interesting polymer-derived system that can be tailored to embody many different properties such as lightweight, electrochemical activity, and high temperature stability. One intriguing property that has not been fully explored is the electrical conductivity for the carbon-rich SiOC compositions. In this study, a carbon-rich SiOC system is created based on the crosslinking and pyrolysis of polyhydromethylsiloxane (PHMS) and divinylbenzene (DVB) mixed precursors. The carbon-rich nature can effectively delay SiOC phase separation and crystallization into SiO2 and SiC during pyrolysis. In an oxidizing air atmosphere, the SiOC materials are stable up to 1000 °C with <0.5 wt% weight loss. Before the onset of electrical conductivity drop at ∼400 °C, the material has electrical conductivity as high as 4.28 S cm−1. In an inert argon atmosphere, the conductivity is as high as 4.64 S cm−1. This new semi-conducting behavior with high thermal stability presents promising application potential for high temperature MEMS devices, protective coatings, and bulk semi-conducting components that must endure high temperature conditions.

 Please wait while we load your content...
Please wait while we load your content...