Electronic alteration of end-on phenyl groups of bis-triazolyl-silanes: electron-transport materials for blue phosphorescent OLEDs†
Abstract
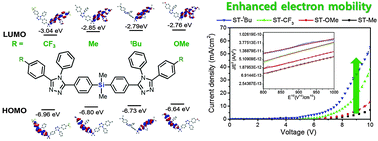
A series of modified bis(4-(4,5-diphenyl-4H-1,2,4-triazol-3-yl)phenyl)dimethylsilane (ST), ST-CF3, ST-Me, ST-tBu, and ST-OMe, were designed and prepared by introducing electron-withdrawing groups or electron-donating groups at the 4-position of the end-on phenyl groups on the triazole ring. Systematic investigations of their thermal-, photophysical-, and electron-transporting (ET) properties were successfully carried out. Depending on the electronic characteristics of the substituents at the end-phenyl group on the triazole, their frontier orbital energy levels were controlled while maintaining energy band gaps. Low-temperature photoluminescence spectra indicate that all the prepared ST moieties maintained high triplet energy states up to 2.85 eV owing to the suppression of electron delocalization by the silicon centre. The electron mobilities of all the compounds were investigated by fabricating electron only devices (EODs). The EOD of ST-tBu showed significantly higher current density than the other analogs. Finally, the EL device utilizing ST-tBu as an electron transporting material in phosphorescent organic light-emitting diodes with bis[(4,6-di-fluorophenyl)-pyridinate-N,C2]picolinate (FIrpic) as a dopant showed an external quantum efficiency of 15.9%.

 Please wait while we load your content...
Please wait while we load your content...