Structure and luminescence properties of Eu2+ doped LuxSr2−xSiNxO4−x phosphors evolved from chemical unit cosubstitution
Abstract
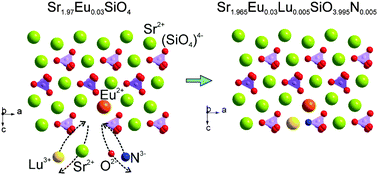
The design scheme of the chemical unit cosubstitution of [Lu3+–N3−] for [Sr2+–O2−] in Sr2SiO4:Eu2+ has been put into practice to discover the new phosphor systems with tunable luminescence properties, and the structures and photoluminescence tuning of yellow-emitting LuxSr2−xSiNxO4−x:Eu2+ phosphors have been investigated. Crystal structures of LuxSr2−x−ySiNxO4−x:yEu2+ samples were resolved using the Rietveld method, suggesting that the as-prepared Sr2SiO4 belonged to monoclinic symmetry (P21/n) of β-phase Sr2SiO4, while Sr1.97Eu0.03SiO4 and Sr1.965Eu0.03Lu0.005SiO3.995N0.005 belonged to orthorhombic symmetry (Pnma) of α-Sr2SiO4. The emission peaks of LuxSr1.97−xSiNxO4−x:0.03Eu2+ phosphors were red-shifted from 563 to 583 nm upon increasing the [Lu3+–N3−] substitution content from x = 0 to x = 0.005, furthermore, the PL emission peaks of Lu0.005Sr1.965−ySiN0.005O3.995:yEu2+ also showed a red-shift from 583 nm to 595 nm with increasing Eu2+ concentration (y = 0.03, 0.07, 0.10 and 0.15), and their corresponding red-shift mechanism has been discussed. The temperature dependent luminescence results further verified that the introduction of [Lu3+–N3−] for [Sr2+–O2−] in Sr2SiO4:Eu2+ can improve the thermal stability of the photoluminescence, which indicated that the LuxSr2−x−ySiNxO4−x:yEu2+ phosphors have potential applications in white light-emitting diodes (wLEDs).


 Please wait while we load your content...
Please wait while we load your content...