Dipolar donor–acceptor molecules in the cyanine limit for high efficiency green-light-selective organic photodiodes†
Abstract
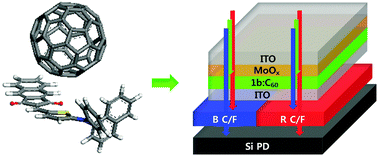
We report on two novel p-type small molecules with a donor–acceptor molecular structure for application to green-light-selective organic photodiodes (OPDs). To achieve the requirement of high light selectivity and sensitivity, an electron-donating aryl amino moiety is combined with two respective electron-accepting heterocycles so that the molecules approach cyanine-like character, characterized by intense and sharp absorption. Molecular stacking is controlled by the addition of bulky aryl functional groups to the main backbone to further control the electrical charge transport properties. With this molecular design, a maximum external quantum efficiency close to 61% (λmax = 550 nm) and a dark-current density below 1.6 nA cm−2 (or specific detectivity D* = 1.19 × 1013 cm Hz1/2 W−1) at an applied reverse bias of 3 V are obtained when mixed with fullerene (C60) in an inverted-structure bulk heterojunction OPD composed of two transparent electrodes. The potential construction of a full-color photodetector or an image sensor is demonstrated by combining the green-light-selective OPD with a silicon photodiode containing solely blue and red color filters in a stacked architecture.

 Please wait while we load your content...
Please wait while we load your content...