Crystallization induced enhanced emission in conformational polymorphs of a rotationally flexible molecule†
Abstract
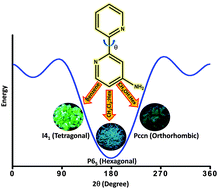
The crystallization of weakly fluorescent 4-amino-2,2′-bipyridine (AMBPY) in solution phase under ambient conditions afforded three fluorescent conformational polymorphs. The marginal increase in the barrier to rotation observed in AMBPY as compared to unsubstituted 2,2′-bipyridine could be attributed to the “buttressing effect” offered by the amino substituent at the meta position. A smaller yet significant difference in energy (0.1–2.6 kJ mol−1) with respect to the global minima facilitates the isolation of AMBPY-I–III polymorphs. A unique nitrogen–nitrogen interaction is observed in two of the polymorphs, namely, AMBPY-I and AMBPY-III, promoted by cooperative C⋯H and N⋯H interactions. A crystallization-induced enhancement (ca. 5–10 fold) in the fluorescence quantum yield of AMBPY polymorphs is observed relative to the solution/amorphous state. Controlling the luminescence properties of molecular solids by tuning their packing arrangements via various interactions is an integral aspect in the construction of novel photo-functional materials.
- This article is part of the themed collection: Shape-Responsive Fluorophores

 Please wait while we load your content...
Please wait while we load your content...