Salicylaldimine difluoroboron complexes containing tert-butyl groups: nontraditional π-gelator and piezofluorochromic compounds†
Abstract
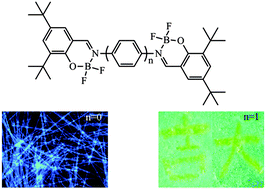
We have synthesized three new salicylaldimine difluoroboron complexes with tert-butyl groups 1B–3B, which are highly emissive in solutions and in solid states. It was found that salicylaldehydehydrazone difluoroboron complex 1B could form organogels in n-hexane and the mixture of petroleum ether/CH2Cl2, and 1D nanoribbons with intense blue emission could be fabricated via the gelation of the nontraditional organogelator 1B directed by balanced π–π interaction. However, the other two salicylaldimine difluoroboron complexes 2B and 3B with a spacer of the benzene ring could not form a gel due to the strong π–π interaction, but exhibited piezofluorochromic behaviors. The as-prepared crystals of 2B and 3B giving blue-green fluorescence could be transformed into the powders emitting yellow light upon grinding, and the emission could recover when the ground powders were heated. It suggested that the reversible piezofluorochromism originated from the transformation between crystalline and amorphous states. It provided a strategy for designing new nontraditional π-gelators and piezofluorochromic materials via tuning the π–π interaction.

 Please wait while we load your content...
Please wait while we load your content...