Size-controlled preparation of gold nanoparticles with novel pH responsive gemini amphiphiles†
Abstract
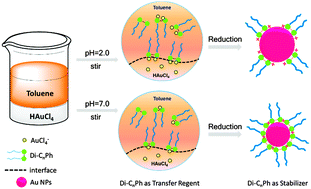
The two-phase synthesis of gold nanoparticles, facilitated by a new family of pH sensitive gemini amphiphiles, Di-CnPh, N,N′-dialkyl-N,N′-di(ethyl-phthalimide) ethylenediamines, simultaneously played the roles as both phase-transfer reagents and stabilizers, was investigated in this study. Di-CnPh had limited solubility in aqueous but could be protonated at water-organic interfaces when the subphase pH is under acidic and neutral conditions, which then served as both the phase transfer agents and stabilizers of Au ions and Au nanoparticles. Size controlled gold nanoparticles were successfully synthesized at acidic and neutral pHs whereas no gold nanoparticles could be detected under basic conditions, which is related to the electrostatic interaction between ligands and metal ions. Meanwhile, the nano size dependence of pH was mainly due to the electrostatic interaction between amphiphiles. Furthermore, the size of Au NPs was highly related to the hydrophobic chain length of Di-CnPh and its concentration in the oil phase as well. The neutral pH condition, the increase in the concentration of amphiphile and the length of the chain favor the formation of smaller size and narrower size distribution of Au NPs.

 Please wait while we load your content...
Please wait while we load your content...