Photoluminescence of CsMI3:Eu2+ (M = Mg, Ca, and Sr) – a spectroscopic probe on structural distortions
Abstract
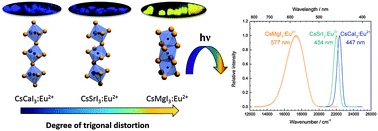
The photoluminescence of Eu2+ ions doped in iodidoperovskites, CsMI3 (M = Mg, Ca, Sr), was studied. Eu2+ occupies the alkaline earth site in each of these compounds. The emission spectra of the doped perovskites are characterized by 4f65d1 → 4f7 transitions of Eu2+ located in the yellow range for CsMgI3 and the blue range for CsCaI3 and CsSrI3, respectively. All excitation spectra provide a well-resolved fine structure arising from different 7FJ (J = 0,…,6) levels of the 4f6 core of the excited state configuration, indicating a weak exchange interaction with the 5d electron. The decay times and temperature dependence of the luminescence were also analyzed. Both CsMgI3:Eu2+ and CsSrI3:Eu2+ show a redshifted emission with respect to CsCaI3:Eu2+. This is explained by trigonal distortions of the [EuI6]4− octahedra in the former two compounds. The energetic order of the resulting crystal field states is justified in terms of the angular overlap model of ligand field theory. This series of Eu2+ doped earth alkaline iodidoperovskites may serve as a textbook example for the detailed understanding of the structure–luminescence relationships of Eu2+ ions, which is very important for tailoring functional materials for respective applications.

 Please wait while we load your content...
Please wait while we load your content...