Synthesis of binary solid solution Cu–Pd nanoparticles by DMF reduction for enhanced photoluminescence properties†
Abstract
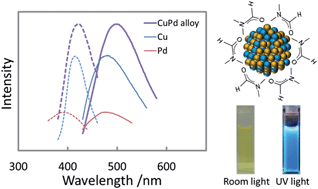
We report the use of a DMF reduction method for straightforward synthesis of binary solid solution Cu–Pd nanoparticles (NPs) over the entire range of composition. The resulting NPs were uniform in size (less than 2.5 nm), tunable in composition, and exhibited photoluminescence properties that were nonlinear in composition. These binary solid solution NPs showed enhanced photoluminescence intensity and quantum yield compared to those of the single-metal NPs and their mixtures. The highest quantum yield of 3.10% for Cu–Pd alloy NPs synthesized using equimolar feeding ratio versus 2.75% for Cu and 0.71% for Pd NPs was obtained. These enhancements make the alloy NPs promising materials for optical applications.

 Please wait while we load your content...
Please wait while we load your content...