Hydrothermal growth and optical properties of Nb2O5 nanorod arrays†
Abstract
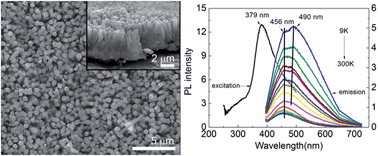
Nb2O5 nanorod arrays were grown on Nb foil through an in situ hydrothermal treatment process using NH4F as the mineralizing agent and H2O2 as the oxidant. The as-prepared Nb2O5 nanorod arrays were well crystallized with a hexagonal structure and a c-axis orientation. The effects of hydrothermal temperature and concentration of NH4F on the growth of the nanorods were investigated. Nb2O5 nanorod arrays are formed by crystal nucleation, oriented growth, followed by orientation attachment. A higher concentration of NH4F accelerates the generation of Nb2O5 nanorods as a result of corroding Nb foil and releasing Nb ions and promotes the oriented growth of Nb2O5 nanorods. The band gap of Nb2O5 nanorod arrays is measured to be about 3.3 eV with a blue light emission located at 456 nm (2.719 eV) and a cyan light emission located at 490 nm (2.53 eV), respectively. The 2.53 eV peak can well be attributed to the donor–acceptor pair (DAP) emission, and the 2.719 eV peak be related to the conduction-band-to-acceptor transitions. There is only one quenching channel for 2.719 eV peak with increasing temperature, which corresponds to the activation energy of about 16.9 meV, according to the theoretical fitting.

 Please wait while we load your content...
Please wait while we load your content...