Electron energy level engineering in Zn1−xCdxSe nanocrystals†
Abstract
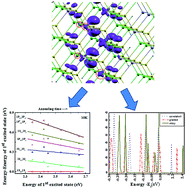
Variation in composition provides an additional degree of freedom in nanocrystals design. In a strategic manner, the amount of Zn across the radius of Zn1−xCdxSe nanocrystals (NCs) is varied, resulting in minimal photoluminescence quenching with temperature, hence assuring the least defect density. Further Zn distribution within NCs is made uniform by annealing. Electron energy levels mapped by optical techniques reveal reduced energy level spacing due to Zn incorporation. The Stokes shift attains a remarkably lower value in alloyed Zn1−xCdxSe NCs. Notably, the alloyed NCs concomitantly exhibit a blue shift in the forbidden gap, but a red shift in higher-energy transitions. First-principles electronic structure calculations show enhanced hybridization of Zn d levels with Se p levels in comparison to that of Cd d levels in homogeneously alloyed NCs, leading to decreasing energy difference between the occupied electron energy levels. Varying the size tunes the optical transitions monotonically, while tuning the composition profile engineers the electron energy levels of NCs.

 Please wait while we load your content...
Please wait while we load your content...