Resonance energy transfer-enhanced rhodamine–styryl Bodipy dyad triplet photosensitizers†
Abstract
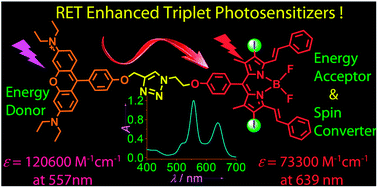
Organic triplet photosensitizers (R-1 and R-2) enhanced with the resonance energy transfer (RET) effect were prepared. Rhodamine was used as an intramolecular energy donor, and iodo-styryl-Bodipy was used as intramolecular energy acceptor/spin converter. Both the energy donor and energy acceptor in R-1 and R-2 give strong absorption in the visible region but at different wavelengths (e.g. for R-1, ε = 120 000 M−1 cm−1 at 557 nm for the energy donor and ε = 73 300 M−1 cm−1 at 639 nm for the energy acceptor). As a result, the photosensitizers show broadband absorption in the visible spectral region. In comparison, conventional triplet photosensitizers contain only one visible light-harvesting chromophore; thus, there is usually only one major absorption band in the visible spectral region. Using steady state and time-resolved spectroscopy, we demonstrated that photoexcitation in the energy donor was followed by intramolecular singlet energy transfer, and then via intersystem crossing (ISC) of the energy acceptor (spin converter), triplet excited states localized on the iodo-styryl-Bodipy were produced, which was confirmed by nanosecond time-resolved transient difference absorption spectroscopy. The organic dyad triplet photosensitizers were used for photoredox catalytic organic reactions to prepare pyrrolo[2,1-a]isoquinoline, and we found that the photocatalytic capability was improved with the RET effect. The dyads were also used as fluorescent stains for LLC cancer cells. Photodynamic effect was observed with the same cells, which were killed on photoirradiation with 635 nm red-emitting LED after incubation with the triplet photosensitizers. Therefore, these photosensitizers can be potentially developed as dual functional theranostic reagents. Using the molecular structural protocol reported herein, organic triplet photosensitizers with strong broadband absorption in the visible spectral region and predictable ISC can be easily designed. These results are useful for the study of organic triplet photosensitizers in the area of organic photochemistry/photophysics, photoredox catalytic organic reactions and photodynamic therapy (PDT).

 Please wait while we load your content...
Please wait while we load your content...