Panchromatic small molecules for UV-Vis-NIR photodetectors with high detectivity†
Abstract
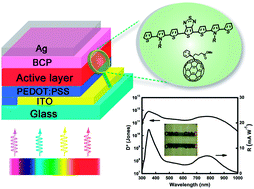
Two donor–acceptor–donor (D–A–D) type low-bandgap small molecules, M1 and M2, with bis(2-thienyl)-N-alkylpyrrole (TPT) as the donor and thieno[3,4-b]thiadiazole (TT) as the acceptor were designed and synthesized. The absorption, transmission, electrochemical, thermal and film properties were studied. The compounds showed panchromatic absorption in the spectral range of 300–1000 nm. Moreover, they also exhibited semi-transparent property in the visible region (400–700 nm). Small molecule photodetectors (SMPDs) based on M1 and M2 were fabricated and studied. For the SMPD with BCP as the hole blocking layer (HBL), a detectivity of 5.0 × 1011 Jones at 800 nm at −0.1 V was obtained, which is among the highest detectivities reported for NIR SMPDs. With a sufficiently thin silver electrode, visibly transparent PDs with an average transmittance of 45% in the visible region were obtained for the first time. The transparent PDs exhibited fairly constant and high detectivity between 1011 and 1012 Jones over a broad spectral range of 300–900 nm. In addition, side chains of the compounds exhibited a great influence on the device performance, which could be assigned to their film absorption coefficient, molecular packing and active layer morphology.

 Please wait while we load your content...
Please wait while we load your content...