Bi-functional mechanism of H2S detection using CuO–SnO2 nanowires†
Abstract
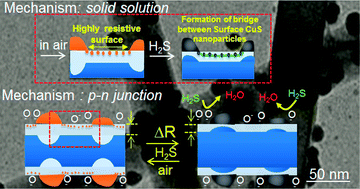
In this study, a bi-functional mechanism is proposed and validated, which may be used to explain all of the reported experimental observations and to predict new sensing control parameters. Fast response and recovery in H2S sensing was then realized by using bi-functional SnO2 nanowires which have been radially modulated with CuO. Firstly, Cu metal nanoparticles were synthesized by applying γ-ray radiolysis. The Cu nanoparticles (attached to the surface of the SnO2 nanowires) were oxidized to the CuO phase by a thermal treatment at 500 °C in air. The H2S sensing characteristics of the CuO-functionalized SnO2 nanowires were compared with those of bare SnO2 nanowires. The results demonstrated that γ-ray radiolysis is an effective means of functionalizing the surface of oxide nanowires with CuO nanoparticles, and CuO functionalization greatly enhanced the ability of the SnO2 nanowires to detect H2S in terms of the response and recovery times. In addition, two control parameters, a 0.5 CuO to SnO2 surface ratio and a sensing temperature range of 80–220 °C, are predicted. The radially modulated nanostructures achieve two functions: (1) the formation and break-away of p–n (CuO–SnO2) junctions, and (2) the formation and dissolution of CuS using CuO–SnO2 solid solutions.

 Please wait while we load your content...
Please wait while we load your content...