When dithiafulvenyl functionalized π-conjugated oligomers meet fullerenes and single-walled carbon nanotubes†
Abstract
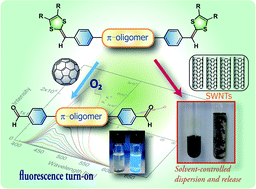
A series of π-conjugated oligomers taking linear and Z-shaped molecular structures were synthesized with electron-donating dithiafulvenyl (DTF) groups functionalized at the terminal positions. Besides significantly enriched electronic and redox properties as disclosed by UV-Vis absorption and voltammetric analyses, these DTF-endcapped oligomers also exhibited a facile reactivity of oxidative C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage sensitized by
C bond cleavage sensitized by

 Please wait while we load your content...
Please wait while we load your content...