Sol–gel preparation of efficient red phosphor Mg2TiO4:Mn4+ and XAFS investigation on the substitution of Mn4+ for Ti4+†
Abstract
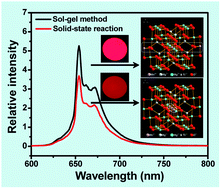
An efficient red phosphor based on the substitution of Mn4+ for Ti4+ in the lattice of Mg2TiO4 was prepared through a sol–gel route. The phosphor was characterized by

 Please wait while we load your content...
Please wait while we load your content...