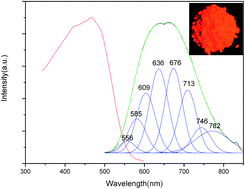
A red-emitting phosphor, Eu2+-activated Ca4(PO4)2O phosphor was synthesized by conventional solid-state reaction. X-ray powder diffraction confirmed the phase formation. The band gap of Ca4(PO4)2O was estimated to be about 4.75 eV from the diffuse reflection spectrum. The excitation and emission spectra, and the concentration dependence of emission intensity were investigated. The results showed that under excitation at 460 nm, the Ca4(PO4)2O:Eu2+ phosphors exhibit strong red emission centered at 665 nm, and the optimum concentration of Eu2+ in Ca4(PO4)2O:Eu2+ is about 0.07 mol per mol Ca2+ ions. The nature of the Eu2+ ion emission in Ca4(PO4)2O, i.e. the main mechanism for concentration quenching was discussed. A white LED was fabricated using a blue 460 nm chip pumped by a phosphor blend of green-emitting Sr2SiO4:Eu2+ and red-emitting Ca3.93(PO4)2O:0.07Eu2+. When the applied current was 20 mA, the white LED has Commission International de I'Eclairage color coordinates of (0.3135, 0.3316) for white light (correlated color temperature = 6446 K) and an excellent color rendering index of 90.5.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...