Facile formulation of alkaline-developable positive-type photosensitive polyimide based on fluorinated poly(amic acid), poly(amic acid), and fluorinated diazonaphthoquinone†
Abstract
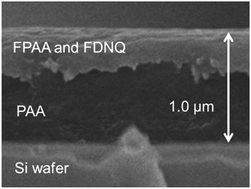
An alkaline-developable, positive-type, and photosensitive polyimide (PSPI) based on fluorinated poly(amic acid) (FPAA), poly(amic acid) (PAA), and fluorinated diazonaphthoquinone (FDNQ-1) as a photoactive compound has been successfully developed. The solution of FPAA, PAA, and FDNQ-1 was spin-coated on a silicon wafer and prebaked. The two phase-separated layers, a thin top layer of FPAA containing FDNQ-1 and a thick bottom layer of PAA, were formed due to the high polarity difference between PAA and FPAA containing FDNQ-1. This phase separation was directly observed in an SEM image. The upper thin layer acted as a photosensitive layer, and a fine pattern was formed in the bottom layer. The PSPI containing PAA (85 wt%), FPAA (15 wt%), and FDNQ-1 (25 wt% to

 Please wait while we load your content...
Please wait while we load your content...