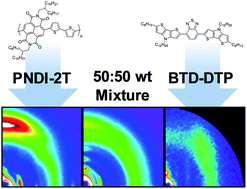
Physical mixtures of organic semiconductors are increasingly used for the development of new materials in thin film, organic electronic applications and their electronic properties are strongly affected by their morphology. Here, we report on studies of blends of an electron-donating small molecule, BTD-DTP, with the electron-acceptor polymer PNDI-2T and the correlations between their thermal behaviour, intermixing and thin film structure. A significant depression of the PNDI-2T melting point (ΔT = 111 °C) is observed upon increasing the small molecule content. Grazing incidence X-ray scattering (GIXS) and scanning probe microscopy (SPM) of thin films of varying composition show an increase in the small molecule crystalline phase and reduction in the crystallite orientation distribution, as the small molecule to polymer ratio reaches ∼50 : 50 wt. The domain sizes of the small molecule and polymer crystalline phases reach a minimum at the 50 : 50 wt ratio as well, suggesting the formation of the phases leads to mutual limitation of their crystalline domain size. Comparison of the bulk and thin film properties shows a divergence in behaviour of the small molecule, which in the bulk exhibits only a monotonic decrease in melting point with addition of polymer, but which has an increase in crystallinity, from 20 to 50 wt% PNDI-2T content.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...