Synthesis and magnetic properties of a 3-D nickel hydroxide capped by succinate†
Abstract
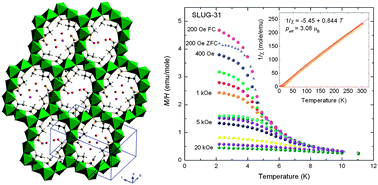
We have successfully synthesized a rare example of an open framework nickel oxide with succinate capping the channels. A honeycomb-like layer of 14-membered rings centered in the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11) plane are connected by vertex-sharing NiO6 octahedra and
11) plane are connected by vertex-sharing NiO6 octahedra and

 Please wait while we load your content...
Please wait while we load your content...