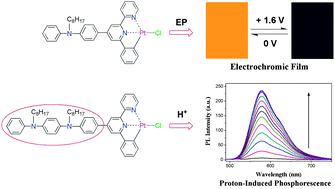
Two cyclometalated Pt(II) complexes, [(L1)PtCl] and [(L2)PtCl] (where HL1 = 4-[p-(N-octyl-N-phenyl)amino]-phenyl-6-phenyl-2,2′-bipyridine and HL2 = 4-{p-[p-(N,N′-dioctyl-N′-phenyl)phenyldiamino]}-phenyl-6-phenyl-2,2′-bipyridine), have been synthesized and characterized by 1H NMR, mass spectrometry (MS) and elemental analysis. The structure of complex [(L1)PtCl] has been revealed by X-ray crystallography. Both of the complexes possess the intense and red-shifted metal-to-ligand charge transfer (1MLCT) (dπ(Pt) → π*(L)) transitions (ε ∼ 104 dm3 mol−1 cm−1). Complex [(L1)PtCl] displays strong phosphorescence (λmax = 597 nm) in CH2Cl2 solution at room temperature and efficient electropolymerization behavior to form an electrochromic film with times of 5.8 s for the coloration step and 5.2 s for the bleaching step, an optical contrast of 70.5%, a black coloration efficiency of 441.4 C−1 cm2 and a bleaching efficiency of 489.1 C−1 cm2. Complex [(L2)PtCl] shows sensitive pH dependent luminescent properties, which are attributed to the inhibition of the intramolecular charge transfer (ICT) process through the protonation of the aniline dimer unit in the cyclometalating ligand.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...