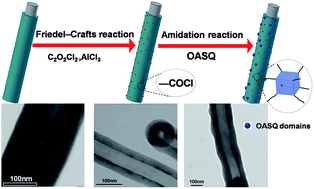
We describe here a facile two-step synthesis of hybrid nanocomposites based on one-dimensional carbon nanofibers (CNFs) decorated with cubic octasilsesquioxane (T8). The functionalization of CNFs with oxalyl chloride, via Friedel–Crafts acylation reaction in the presence of aluminum chloride, was first demonstrated as an efficient way to introduce carboxyl acid groups onto the nanofiber sidewalls. Octa-aminophenylsilsesquioxane (OASQ) was then covalently attached to the carboxylated nanocarbon CNF–(COOH)n through amide linkage. The CNFs were characterized by a set of material characterization methods including FTIR, Raman, solution 1H NMR spectroscopy, Auger electron spectroscopy (AES), thermogravimetric analysis and transmission electron microscopy (TEM) at each step of the functionalization. The results clearly show that the formation of the conjugated carboxylic acid moiety, due to the nature of the substitution, occurs at defect sites on the carbon nanofibres and the carboxylation degrees can be controlled by variation of the reaction conditions. High-resolution TEM observations and physisorption data reveal that OASQ domains are uniformly distributed on the surface with an average particle size of 5 nm and T8 cage probably remains intact during the decoration process.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...