An anthraquinone-based highly selective colorimetric and fluorometric sensor for sequential detection of Cu2+ and S2− with intracellular application†
Abstract
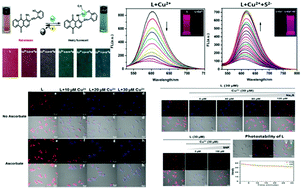
2-(1-Amino-2-anthraquinonyliminomethyl)phenol (L) was facilely prepared as a spectroscopic probe by one step condensation between 1,2-diaminoanthraquinone and salicylaldehyde. The complexation of Cu2+ ions with L through 1 : 1 chelation resulted in a rapid pink-to-blue color change and significant quenching of the fluorescence at 604 nm in 1 : 1 THF : Tris–HCl buffer. The subsequent addition of S2− to this solution regenerated the initial spectrum of L as a result of L being released from the L–Cu2+ complex through a displacement mechanism, which makes L a dual-channel sensor for the naked eye detection of Cu2+ and S2− ions. The system has high selectivity toward Cu2+ and S2− ions even in the presence of other commonly coexisting ions and the detection limits were found to be 8.95 × 10−8 M and 1.36 × 10−7 M, respectively. Applicability of L to detect Cu2+ ions and S2− ions in tap water has been demonstrated. Paper strips were fabricated for the detection of Cu2+ and S2− by the colorimetric method. Furthermore, L has been successfully applied for cell imaging studies.


 Please wait while we load your content...
Please wait while we load your content...