An activatable anticancer polymer–drug conjugate based on the self-immolative azobenzene motif†
Abstract
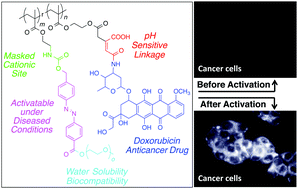
Triggered cellular uptake of a synthetic graft copolymer carrying an anticancer drug is achieved through self-immolation of the side-chain azobenzene groups. In this concept, the conjugate is initially chemically neutral and does not possess cell-penetrating function. However, upon cleavage of the azobenzene moieties, a cascade process is initiated that ultimately reveals an ammonium cation in the vicinity of the polymer backbone. Hence, self-immolation results in the transformation of the neutral polymer chain into a polycation. This structural transformation allows the conjugate to be taken up by the cancer cells through favorable electrostatic interactions with the negatively charged phospholipid components of the cell membrane. Once inside the cells, the polymer releases covalently attached doxorubicin in a pristine form through a low pH activated release mechanism. The significance of this approach lies in the sensitivity of the azobenzene group to hypoxic conditions and to the enzyme azoreductase that is secreted by the microbial flora of the human colon and suggests a pathway to targeted drug delivery applications under these conditions.


 Please wait while we load your content...
Please wait while we load your content...