Conformation and self-assembly changes of isomeric peptide amphiphiles influenced by switching tyrosine in the sequences†
Abstract
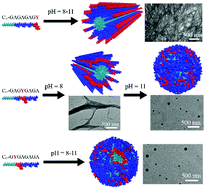
Self-assemblies of peptide amphiphiles feature unique structures, high biocompatibility, and potential for various applications, and have attracted increasing interest in supramolecular chemistry, protein science and polymer science. In this paper, isomeric peptide amphiphiles derived from lauric acid and silk fibroin-based peptides with different amino acid sequences (GAGAGAGY, GAGAGYGA, GAGYGAGA and GYGAGAGA) are investigated systematically to figure out the predominant endogenous and exogenous factors for their assembly in aqueous solution. With the position of tyrosine (Y) in the peptide segment gradually moving towards the alkane tails, the assembled peptide amphiphiles substantially change their secondary structures from the β-sheet to the disorder dominant one under neutral pH conditions, because the increase of steric hindrance induced by the position change of Y disturbs the hydrogen bonds relevant to the formation of β-sheets of (GA)n. Strong alkaline conditions are able to accelerate such a conformational change, due to the synergy of destruction of hydrogen bonds, the steric hindrance effect and electrostatic repulsion. As a consequence, the assembled peptide amphiphiles alter their nanostructures in aqueous solution from well-defined nanofibers to nanospheres with varying sizes. Therefore, it is summarized that the location of Y rather than the other effects such as pH value, etc. plays an essential role in the assembly of our isomeric peptide amphiphiles, which sheds light on the design of various isomeric peptides/peptide amphiphiles for their aggregation as well as potential functionality.


 Please wait while we load your content...
Please wait while we load your content...