Polymer cloaking modulates the carbon nanotube protein corona and delivery into cancer cells†
Abstract
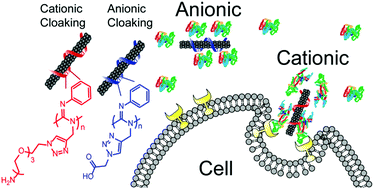
Carbon nanotube-based molecular probes, imaging agents, and biosensors in cells and in vivo continue to garner interest as investigational tools and clinical devices due to their unique photophysical properties. Surface chemistry modulation of nanotubes plays a critical role in determining stability and interaction with biological systems both in vitro and in vivo. Among the many parameters that influence the biological fate of nanomaterials, surface charge is particularly influential due to direct electrostatic interactions with components of the cell membrane as well as proteins in the serum, which coat the nanoparticle surface in a protein corona and alter nanoparticle–cell interactions. Here, we modulated functional moieties on a helical polycarbodiimide polymer backbone that non-covalently suspended the nanotubes in aqueous media. By derivatizing the polymer with either primary amine or carboxylic acid side chains, we obtained nanotube complexes that present net surface charges of opposite polarity at physiological pH. Using these materials, we found that the uptake of carbon nanotubes in these cells is highly dependent on charge, with cationic nanotubes efficiently internalized into cells compared to the anionic nanotubes. Furthermore, we found that serum proteins drastically influenced cell uptake of the anionic nanotubes, while the effect was not prominent for the cationic nanotubes. Our findings have implications for improved engineering of drug delivery devices, molecular probes, and biosensors.
- This article is part of the themed collection: Carbon Nanostructures in Biology and Medicine


 Please wait while we load your content...
Please wait while we load your content...