Synthesis and structure of iron- and strontium-substituted octacalcium phosphate: effects of ionic charge and radius†
Abstract
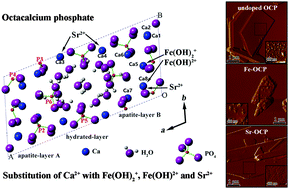
Octacalcium phosphate (OCP) has received intensive research focus as a main component of bone substitute materials due to its highly osteoconductive and biodegradable characteristics. In this work, OCP was synthesized using chemical precipitation methods. Biologically relevant iron ions (Fe3+) and strontium ions (Sr2+) which have different ionic charges and radii were successfully introduced into OCP crystal structure, and their effects on the formation, phase components and structure of OCPs were investigated. The incorporation of Fe3+ and Sr2+ led to lattice expansion of OCP. Both ionic substitutions had slight effects on the morphology and microstructure of typical plate-like OCP crystals. In particular, nanosized particles containing rich Fe were deposited on the surface of plate-like Fe3+-substituted OCP crystals, which confirmed the influence of iron substitution on the corresponding crystal surface nature. This work highlights the different replacements of complex Ca sites by Fe and Sr in the apatite layers and hydrated layers of OCP crystal structure, which gives more possible accounts for foreign trivalent and divalent cations.

 Please wait while we load your content...
Please wait while we load your content...