Enzymatic electrochemical glucose biosensors by mesoporous 1D hydroxyapatite-on-2D reduced graphene oxide†
Abstract
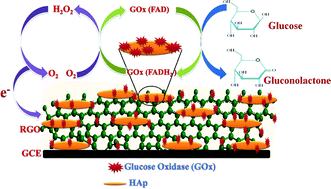
A novel hydrothermal process was used for the preparation of hydroxyapatite (HAp) nanorods on two-dimensional reduced graphene oxides (RGO). The hydrothermal reaction temperature improves the crystallinity of HAp and partially reduces graphene oxide (GO) to RGO. The crystalline structure, chemical composition and morphology of the prepared nanocomposites were characterized by using various analytical techniques. Nanorods of HAp with a diameter and length of ∼32 and 60–85 nm were grown on basal planes and edges of the layered RGO sheets. The estimated specific surface area and pore-size distribution are 120 m2 g−1 and 5.6 nm, respectively. We also report the direct electrochemistry of glucose oxidase (GOx) on 1D HAp-on-2D RGO nanocomposite-modified glassy carbon electrode (GCE) for glucose sensing. The electrocatalytic and electroanalytical applications of the proposed RGO/HAp/GOx-modified GCE were studied by cyclic voltammetry (CV) and amperometry. The increased electron rate constant of 3.50 s−1 was obtained for the modified GCE. The reported biosensor exhibits a superior detection limit and higher sensitivity ca. 0.03 mM and 16.9 μA mM−1 cm−2, respectively, with a wide linear range of 0.1–11.5 mM. The tremendous analytical parameters of the reported sensor surpass those of related modified electrodes and are promising for practical industrial applications.

 Please wait while we load your content...
Please wait while we load your content...