Optical properties of hemicyanines with terminal amino groups and their applications in near-infrared fluorescent imaging of nucleoli†
Abstract
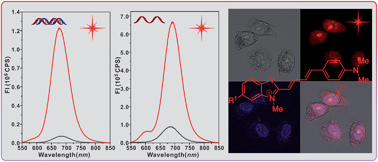
Long wavelength chromophores are of interest for material and biological applications. Six hemicyanine dyes (1a–f) constructed with oxazolo-pyridinium, indolium, benzooxazolium, or benzothiazolium groups and terminal anilino groups with multiple methylenes were prepared. They all exhibited fluorescence from 600 to 850 nm with emission maxima in the near-infrared region. The Stokes shifts became larger in more polar solvents, likely as a result of the greater dipole moments and geometry relaxations in the excited states as suggested by (TD)DFT calculations. The hemicyanine dye with the benzothiazolium group (1d) exhibited the best photostability and thermal stability among those tested. The potential application of 1d as a nucleic acid-staining fluorophore was evaluated. Fluorescent responses were observed from 575 to 850 nm when 1d was titrated with commercially available DNA and RNA. Dye 1d was also used in the selective fluorescent staining of RNA in live HeLa, KB and V79 cells. The results indicated that 1d can be used as a near-infrared fluorescent probe for nucleolus imaging in live cells.

 Please wait while we load your content...
Please wait while we load your content...