Self-assembled pH-responsive films prepared from mussel anchoring threads†
Abstract
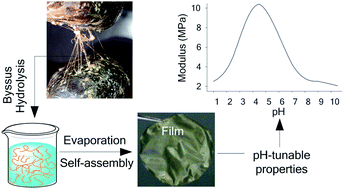
The byssus is a series of collagen-rich fibers securing mussels to surfaces. The complex but elegant heterogeneous assembly of the various proteins in the threads is responsible for their remarkable mechanical properties combining strength and extensibility. Along with the well-known biocompatibility and biodegradability attributed to collagen-based materials, these mechanical properties are highly desirable to produce biomaterials for soft tissue engineering and drug delivery applications. In order to replicate the byssus natural features and properties, we prepared a soluble byssus protein hydrolyzate (BPH) that can generate water-insoluble self-standing films. Atomic force and scanning electron microscopy revealed the presence of self-assembled collagen-like fibrils at the surface of the films. Infrared spectroscopy analysis of the film formation showed that insolubility is caused by the self-assembly of polypeptides from the hydrolyzate into antiparallel β-sheets, aggregated β-strands and collagen triple-helix structures. The mechanical properties and water swelling measurements on the films can be reversibly pH-modulated by modifying the electrostatic interactions between the ∼30 mol% of charged amino acids. Optimal mechanical properties and minimum swelling are obtained at the isoelectric point (pH 4.5). Higher or lower pH treatment reversibly decreases their stiffness and strength and increases their swelling ratio. Altogether, our results show that byssus proteins are an interesting sustainable feedstock for preparing new solid-state pH-tuneable biomaterials.

 Please wait while we load your content...
Please wait while we load your content...