Polycyanoacrylate porous material for bone tissue substitution†
Abstract
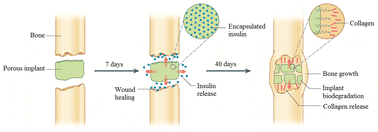
A proof of concept study has been conducted for the design of a porous biodegradable material containing nanocapsules and two actives with independent release-bimodal drug-eluting implants. Completely safe synthetic material free from risk of prion and virus contamination was tested in vivo, and a method for controlling the rate of biodegradation of poly-2-cyanoacrylic polymer was developed. Novel perfluorinated 2-cyanoacrylic esters have been applied for the chemical modification of polyethyl-2-cyanoacrlylate copolymers. Internal imide-cycle formation has been used to retard the rate of enzymatic hydrolysis of the 2-cyanoacrylic copolymer main chain.

 Please wait while we load your content...
Please wait while we load your content...